Abstract
Introduction: Venous thromboembolism (VTE) is a significant adverse event in adults receiving pegaspargase (PEG) for acute lymphoblastic leukemia (ALL). PEG increases VTE risk by depletion of antithrombin III (AT). Heparin requires adequate AT for anticoagulation. Younger adults with T-cell ALL receiving prednisone may be particularly at risk. Retrospective series (most with L-asparaginase) suggest AT supplementation may decrease VTE, however prospective data in adults beyond induction is limited while the optimal dose of AT remains undefined and varies across series. We reviewed adults at our institution who received PEG for ALL to assess the incidence of VTE within our AT supplementation practice. Laboratory and cost data for AT repletion were also analyzed.
Methods: Adults who received PEG for ALL between 11/2015 and 7/2018 were retrospectively identified. Institutional recommendations were to supplement AT if serum AT < 60% following PEG for at least the first 2 courses (induction/consolidation). AT levels were assessed twice weekly until normalized. AT supplementation following additional cycles was recommended for all patients receiving therapeutic anticoagulation. Pharmacists calculated the AT dose using a repletion factor of 80-120%, rounded to the nearest vial. After VTE, patients received therapeutic enoxaparin throughout all remaining PEG doses, with enoxaparin held only if platelets < 50,000/mcL or for procedures. After 3/2018, all patients receiving PEG also received enoxaparin prophylaxis when platelets >30,000/mcL. A retrospective analysis was done to assess the incidence of VTE. Secondary endpoints included an assessment of VTE risk factors, ability to achieve therapeutic AT levels with supplementation and to characterize drug therapy costs with AT supplementation.
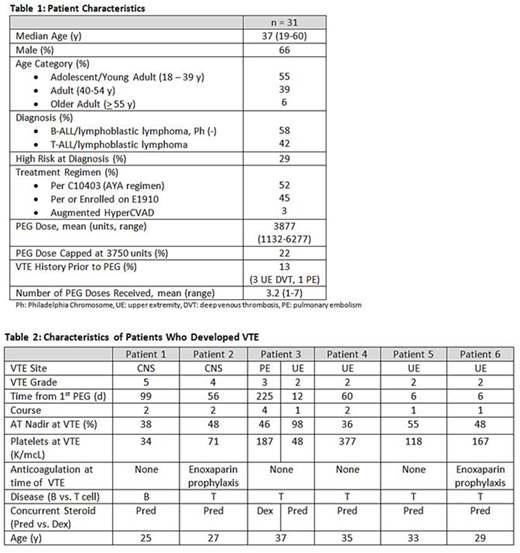
Results: Thirty-one patients (30 newly diagnosed, 1 in relapse) with ALL received ≥ 1 dose of PEG followed by AT supplementation. Seventeen of 31 patients were adolescent/young adults (AYA) and 13/31 had T cell ALL. Additional patient characteristics are summarized in table 1. The incidence of VTE was 19%, with 7 VTEs identified in 6 patients. Two patients developed CNS thrombosis (1 fatal), 1 had a pulmonary embolism, and the remainder were upper extremity VTE. Six of 7 VTE occurred during the first two courses at a mean of 66 days (range 6-225) following the first PEG dose. Patients with VTE had a median platelet count of 118/mcL (range 34-377) and a mean AT nadir of 53% (36-98) within 72 hours of VTE. Two of 7 events occurred despite enoxaparin prophylaxis. Five of 6 (83%) patients with VTE had T-ALL; which was more common in the VTE vs. no-VTE group (p = 0.01). The incidence of VTE within the T-ALL group was 38%. Patients with VTE were all AYA and were younger than those without VTE (median 31 vs. 42 years, p = 0.06). Patients with VTE received a higher mean PEG dose than patients without VTE (4589 vs. 3504 units, p < 0.0001), reflective of the more aggressive dosing in the AYA regimen. Six of 7 VTEs occurred during a course containing prednisone (p = 0.08 vs. dexamethasone). AT nadirs during cycles with VTE were similar to cycles without VTE. No clinically significant bleeding occurred. Characteristics of patients with VTE are summarized in table 2.
Overall the mean time to AT nadir was 11 days. Therapeutic AT (> 60%) following supplementation occurred 56% of the time. Most AT doses (89%) were calculated with a correction factor of 80-89%. The probability of obtaining a therapeutic AT increased when a higher repletion factor (> 90%) was used (76% vs. 52%, p = 0.06). Patients received a mean of 1.9 (0-6) doses of AT per PEG dose, and a mean of 5.9 (1-21) AT doses throughout treatment. The mean AT supplementation cost per PEG dose was $11,663 with 186 doses administered ($3.22/unit).
Conclusions: VTE occurred in 19% of patients receiving AT supplementation following PEG, with 2/7 events involving the CNS. The risk of VTE was greatest in younger adults with T-ALL receiving concurrent prednisone and higher doses of PEG. AT levels were low at the time of VTE in most patients, however nadirs were similar compared to courses not complicated by VTE. Routine or augmented VTE prophylaxis and a higher AT repletion goal (> 90%) may further limit VTE risk but given the cost and patient inconvenience, prospective evaluation is needed to confirm the benefit.
Frey:Servier Consultancy: Consultancy; Novartis: Consultancy. Perl:Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy; Actinium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Arog: Consultancy; Astellas: Consultancy; NewLink Genetics: Membership on an entity's Board of Directors or advisory committees. Porter:Genentech: Other: Spouse employment; Novartis: Other: Advisory board, Patents & Royalties, Research Funding; Kite Pharma: Other: Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal